Since 1966
Learn more
If you wish, we can design special products for you in line with your expectations and produce effective and reliable solutions for your needs.
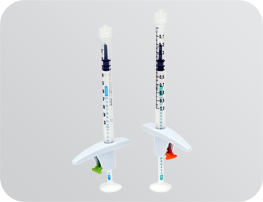
The use of an accurate dosage syringe is beneficial to both experienced doctors as well as to professionals who inject less frequently.
Learn more
These unique plastic syringes are more chemically resistant than rubber-tipped syringes and have a positive safety stop to help prevent accidental spills.
Learn more
Suitable for blow-fill-seal production, medical and pharma grade materials, Cleanroom production and assembly and beta port containers/bags.
Learn more
Dry powder inhalers are commonly used for the delivery of inhaled medications, and should provide consistent, efficient dosing and be easy to use correctly.
Learn more
There are various technologies available within the inhalation category. One popular, proven and convenient system is the capsule-based dry powder inhaler.
Learn more
We offer large range of medical components and pharmaceutical componets to create a customized kits under our or your label.
Learn moreOver Years of Experience
Teammates
Patent Development
Million Product Capacity

Our company, which implements the zero waste project in its entirety, in waste management; By 2023, we have reduced the use of plastic materials used in the packaging of inhaler devices and achieved an annual improvement of 13% in packaging use.
We regularly collect e-waste and donate the revenues from these wastes to TODEV (Turkish Autistic Support and Education Foundation). At the same time, end-of-life batteries are collected, collected and forwarded to the TAP Association (Portable Battery Manufacturers and Importers Association). We recycle the leftover parts from the production processes and reuse them in different areas.